MRDD
Overview
This module estimates maximum recommended daily dose (MRDD) that can be used for humans in clinical trials. Description and data compilation of MRDD values were taken from the publication by Contrera J.F. et al. Regul Toxicol Pharmacol. 2004; 40(3):185-206 [1] Prediction model of MRDD values is based on PLS and binomial regression analysis of Contrera et al. MRDD data set using fragmental descriptors. It also utilizes the Model Applicability Domain assessment methodology allowing the calculation of the Reliability Index for every prediction (see more information about Trainable Models).
Note: Estimation of MRDD is only approximate and can not be used for selection of starting dose in clinical trials.
Interface
Results:
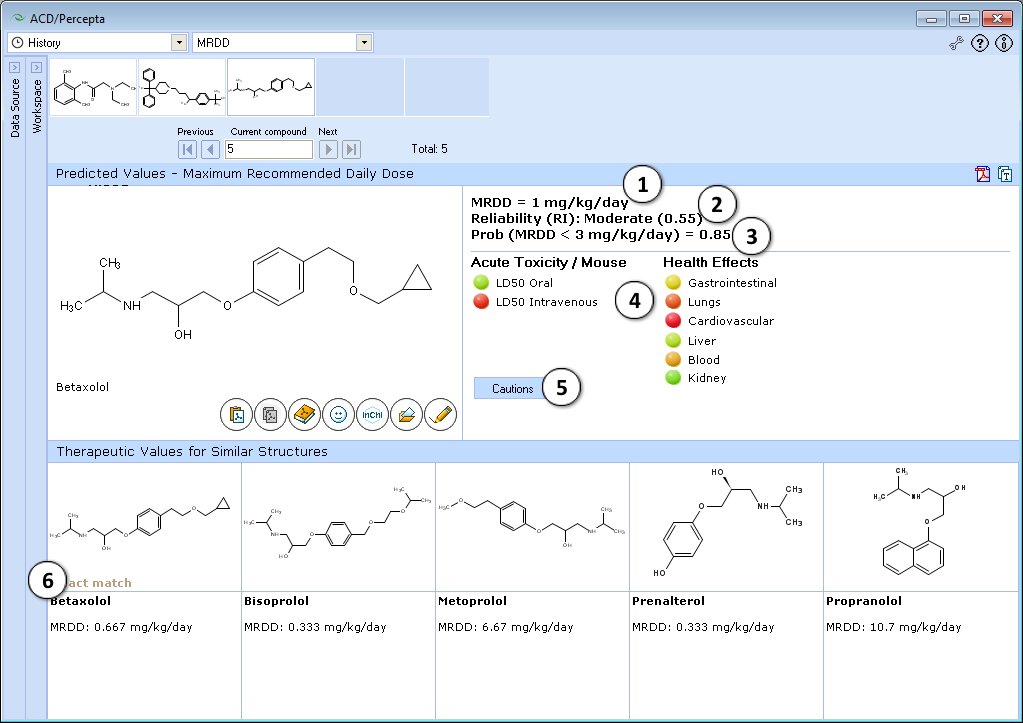
- Estimated maximum recommended daily dose in humans (mg/kg/day) after oral administration
- Indication of the prediction reliability along with the Reliability Index value
- Estimated probability that drug candidate can be used only at low doses
- Displays possible explanation that restrict the use of drug at higher dose: predicted high acute toxicity (undesirable property is red) or high probability of health (adverse) effects on different organs
- The button appears in case of unreliable prediction. The unreliable results are for compounds that are dissimilar to training set, are used at very low doses and predicted to be toxic. Click the button to see more detail explanation of cautions for tested compound.
- MRDD values for up to 5 similar drugs from Contrera et al. data set
Cautions:
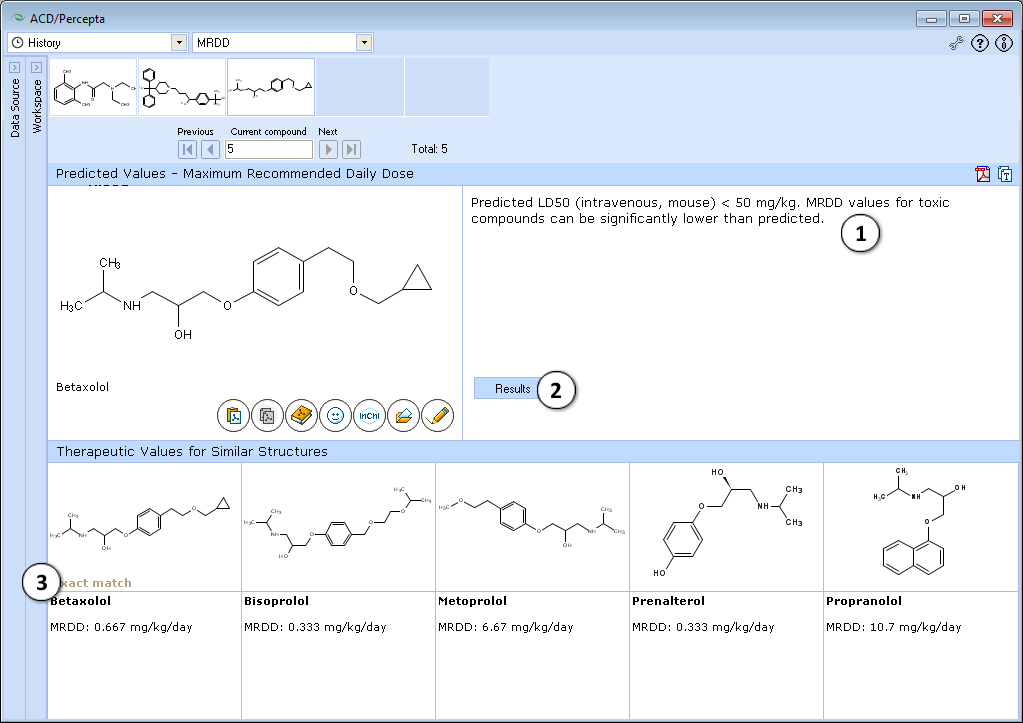
- Text of the caution
- Switch to the results of MRDD prediction
Note: Prediction reliability classification according to Reliability Index (RI) values:
- RI < 0.3 – Not Reliable,
- RI in range 0.3-0.5 – Bordeline Reliability,
- RI in range 0.5-0.75 – Moderate Reliability,
- RI >= 0.75 – High Reliability
Technical information
Training set size: 1,313
Internal validation set size: 143
Main sources of experimental data:
- Contrera, J.F. et al. Regul. Toxicol. Pharmacol. 2004, 40(3): 185-206
Internal Validation
MRDD is one of the many parameters evaluating the potential of a compound to cause acute toxicity or long-term adverse effects, i.e., it is a very complex property depending on a variety of complicated mechanisms. As a result, the only available MRDD dataset published by Contrera and coauthors is nowhere near enough for the development of a robust global quantitative model. As a result this model should be treated only as a very rough estimation, and therefore its validation wasn't emphasized as the results can hardly lead to any relevant conclusions. In this respect the qualitative model indicating the probability that compound's MRDD does not exceed 3 mg/kg/day can be of greater practical value as it can be indicative of potential safety problems associated with the query compound. Internal validation results for this model are provided in the below table. In any case it should be stressed that any estimations provided within ACD/MRDD module are only approximate and can not be used for selection of starting dose in clinical trials.
| Subset | Coverage of the entire internal validation set (N=143) |
Observed* | Calculated probability (p) | |||
|---|---|---|---|---|---|---|
| >0.5 | <0.5 | |||||
| RI > 0.3 N = 143 |
|
True | 17 (11.9%) |
9 (6.3%) | ||
| False | 41 (28.7%) |
76 (53.1%) | ||||
| Accuracy |
| |||||
| Sensitivity |
| |||||
| Specificity |
| |||||
* - True means that the compound is recommended to be used only at very low doses (MRDD < 3 mg/kg/day).